When Is Ethics Approval Required?
When is Ethics Approval Required?
Any study which involves systematic investigation, including research development, testing, and evaluation, and are designed to develop or contribute to generalizable knowledge is considered research and will require NHG Health DSRB review and approval if it involves patients, staff, premises or facilities of NHG Health institutions and all other institutions under the oversight of NHG Health DSRB.
-
Learn more about using the Ethics and Compliance Online System (ECOS) for ethics submissions to the DSRB here.
Why is DSRB Approval Required?
All research proposals that intend to enroll human subjects must meet certain criteria before study procedures can be initiated. The criteria are based on the principles of respect for persons, beneficence and justice as discussed in the Belmont Report.
The Principal Investigator is strongly encouraged to submit their application well before the deadline for submission to allow some time for the DSRB to check for any missing documents for information. The DSRB relies solely on the documentation submitted by the Principal Investigator for review.
Which Research Activities requires DSRB Approval?
Types of studies that may require DSRB Approval:
• Case series (3 or more subjects)
• Database studies
• Tissue Repositories
Types of studies that may not require DSRB Approval:
• Case reports
• Outbreak investigations
• Disease Management
• Infection Control
• Quality Assessment & Improvement (QA/QI)*
• Studies involving Anonymised Data and/or Human Biological Material (HBM)#
*QA/AI Activity Checklist: The QA/QI Activity Checklist may be used to determine if a QA / QI study requires DSRB review. Where the response to all questions in the checklist is “No”, and where there is no intention to share the information with others (i.e. contributing to generalizable knowledge) at the onset of the study, the QA / QI study will not be subject to DSRB review. Download the QA/QI Activity Checklist here.
When in doubt whether an activity requires DSRB review and approval, the Principal Investigator may contact the DSRB and provide a summary of the proposal for a preliminary assessment. The Principal Investigator may submit an application for the DSRB to review. The DSRB will issue a notification to the Principal Investigator if the DSRB determines that the proposal does not require review/approval.
Resources: See the NHG Health Investigators' Manual (IM Edition 5, Chapter 1, Section 1.5, Page 12-13) here.
#Declaration of Use of Anonymised Data and/or Samples for Research’ Form:
Studies involving anonymised data and/or HBM will not require review by DSRB as these studies do not meet the definition of human subject research, if there is no use of identifiable private information or identifiable biospecimens, and no interactions or interventions with human participants in the study.
The ‘Declaration of Use of Anonymised Data and/or Samples for Research’ form may be used to evaluate whether the research involving anonymised data and/or HBM qualifies as ‘Not Human Subject Research’. Researchers can use the form to seek endorsement from their DR and IR to proceed with their research. Researchers should retain the endorsed form as proof of institutional review. Download the declaration checklist here.
However, institutions may request researchers to submit their research applications on ECOS when there is ambiguity whether the study requires DSRB review and approval. The workflow for submission of such studies is shown in Figure 1 below.
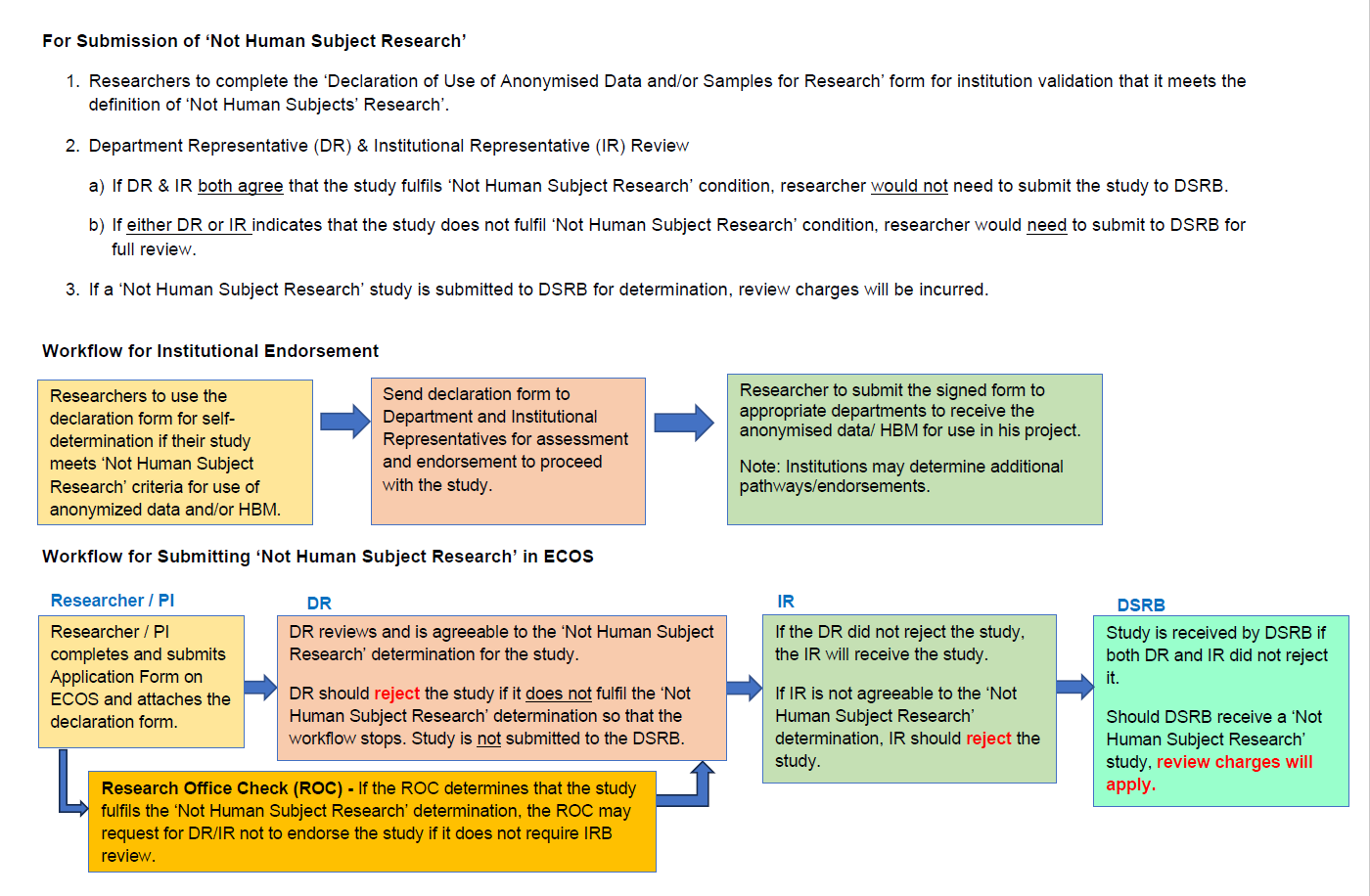
Figure 1 Submission of "Not Human Subject Research"
Resources: See the NHG Health Investigators' Manual (IM Edition 4, Chapter 1, Section 1.5, Page 14-15) here.
What are the criteria for DSRB Approval?
The DSRB will consider the following elements of review which are also the minimum criteria for DSRB approval for new applications, study amendments and continuing review:
• Risks to subjects are minimized by using procedures which are consistent with sound research design, do not unnecessarily expose subjects to risks, and when appropriate already being performed for diagnostic or treatment purpose.
• Risks to subjects are reasonable in relation to anticipated benefits, if any, to subject and the importance of the knowledge that may reasonably be expected to result.
• Selection of subjects is equitable. In making this assessment, the DSRB will take into action account the following:
o The purpose of the research
o The setting in which the research will be conducted.
o Special problems of research involving vulnerable populations (i.e. children, prisoners, pregnant women, mentally disabled persons or economically or educationally disadvantaged persons.)
• Informed consent will be sought from each prospective subject or the subject’s legally acceptable representative, in accordance, and to the extent requirements for Research Involving Children.
• Informed consent will be appropriately documented.
• When appropriate, there are adequate provisions to protect the privacy of subjects and to maintain the confidentiality of data.
• When some or all of the subjects are likely to be vulnerable (i.e. children, prisoners, pregnant women, mentally disabled persons or economically or educationally disadvantaged persons), to coercion or undue influence additional safeguards have been included in the study to protect the rights and welfare of these subjects.
Resources: See the DSRB Review Requirements & Process Guide here.

